The compelling case for ready-to-use stain kits in pathology lab
.webp)
What is the primary role of the biomedical scientist (BMS) in a pathology laboratory preparing patient samples for analysis?
In our years’ working with biomedical scientists, we know they need to prepare samples both quickly and efficiently, while maintaining consistent quality and accuracy.
And this is often under the pressures of time, budget and having enough skilled staff to handle the workload of a busy lab.
So, how does having access to pre-formulated and validated stains and stain kits make a difference?
Stains – one of the three pillars
If the first two pillars of histology include the processing and sectioning of a tissue specimen, the third pillar is the staining process: one of the essential elements before presenting the sample to a pathologist for diagnosis.
Therefore, a BMS needs the stains they use to work first time, work efficiently and be consistently reliable and of high quality.
While tradition sometimes dictates that technicians formulate their own stains in the lab, this can bring its own share of problems: along with tying up busy staff with a manual process, to be compliant with ISO5189 the results must be validated in house for every component prior to use.
In addition, always having the right chemicals to hand means sourcing, validating and storing the raw materials, plus awareness of expiry dates to meet tightening regulations, including UKAS accreditation of ISO standards.
The idea that formulating stains from scratch in the lab is a cost-effective method is, more often, a false economy. The better option is using pre-formulated stain kits.
In the data below, we have compared the cost of a stain kit to that of purchasing the individual components required to make each kit. Also, the shelf life of the stain kits and separate raw materials are the same – and each subject to ISO15189 shelf-life requirements.
Though our information does not include the cost of sourcing, purchasing, storing, making or validating each component used in each stain kit, we believe it demonstrates clearly the benefits of switching to pre-validated stain kits, and that costs can be “recouped” in a very short period of time.
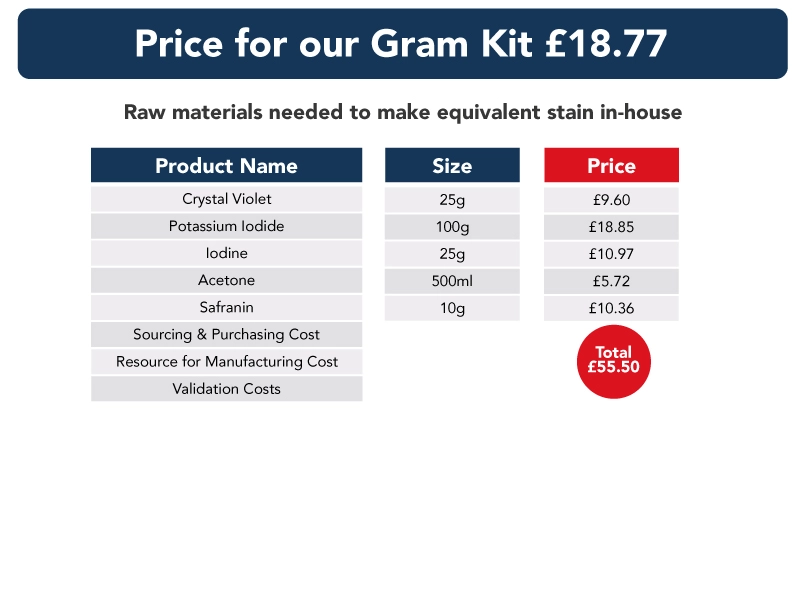
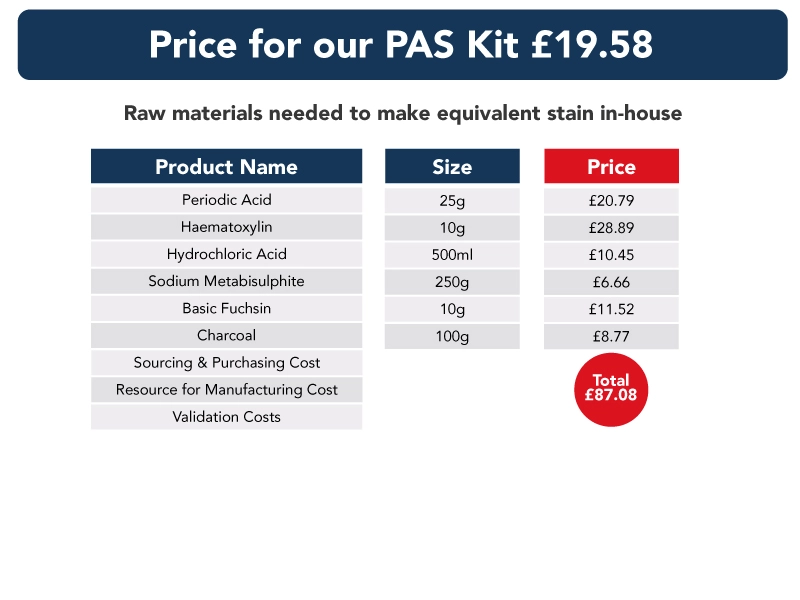
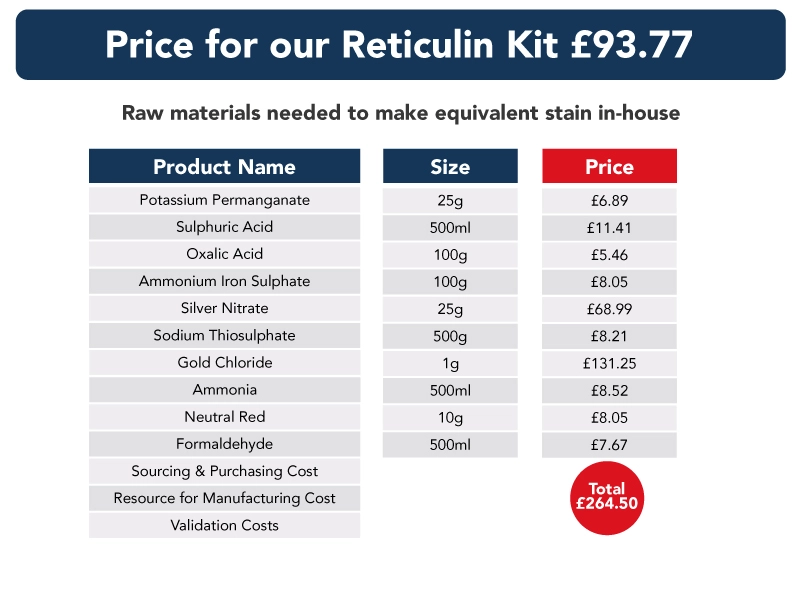
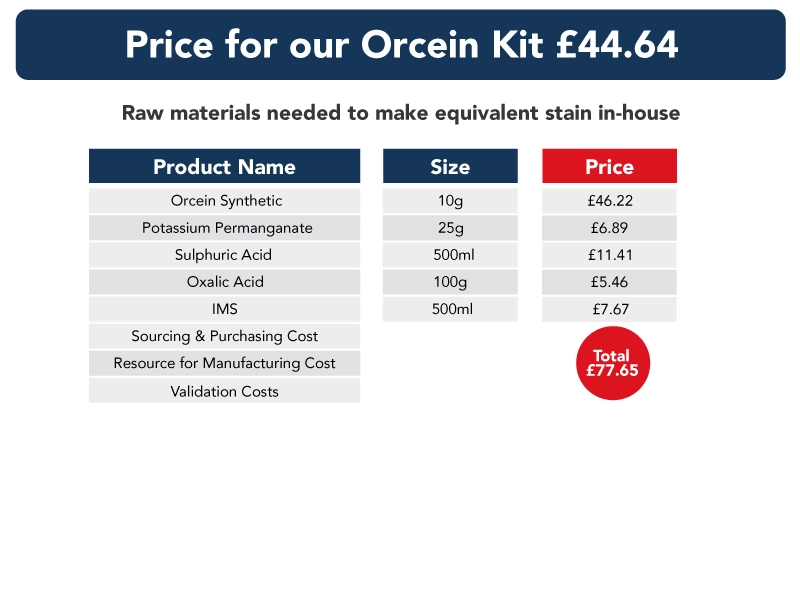
Ready to use stain kits – a guarantee of quality
Employing ready-to-use and validated stain kits in the lab saves the modern BMS time and provides consistent and reliable results within proven protocols.
Atom Scientific’s stain kits, independently assessed and scored by UK NEQAS CPT, and tested in our QC Lab before supply, gives the external, pre-validated quality assurance that any BMS can rely on.
Our kits offer complete traceability of all batches and raw materials, so labs can be sure there is a robust audit trail if needed. We also offer full technical support to ensure you get the best out of our kits.
Some of Atom Scientific’s stain kits contain up to 20+ separate raw materials in one, which removes the requirement for labs to source and store the multiple raw materials used in some stains.
The years of investment we’ve made in developing stain kits has resulted in Atom Scientific achieving several quality standards in 2022 alone. We are the only UK manufacturer of diagnostic stain kits to comply with the recent EU regulation 2017/746 covering In Vitro Diagnostic Medical Devices for diagnostic use (IVDR) as well as research. This sits alongside the IVDD registration that Atom Scientific obtained several years ago.
Our stain kits and biological stains, whilst retaining their CE mark outside of the UK, have also this year attained the new UKCA mark for general In Vitro Diagnostic (IVD) devices, which complies with various ISO standards and replaces the former CE mark for the UK markets, since the UK exited the EU.
Reaching these standards for stain quality means we can play a part in our laboratory customers’ own compliance with quality management systems, such as ISO15189 for quality and competence in medical laboratories.





